hepatitis c testing ultimate guide
Did You Know Only 21% of people know they have HCV? How testing plays a role in ending hepatitis C?
Hepatitis C is a viral infection in the liver caused by the hepatitis C virus, or HCV. It is spread through contact with blood from an infected person. Today, most people become infected with the virus by sharing needles or through equipment used to prepare or inject drugs. However, it can also be spread through birth from an infected mother to child, through sexual contact, sharing personal items contaminated with blood such as razors and toothbrushes, unregulated tattooing, and some health care procedures such as injections, infected blood transfusions (very rare in Canada and the US, read more about the risk of diseases from blood transfusions a previous blog post), and needlestick injuries in healthcare settings.
The immediate period following infection is called the acute phase and lasts approximately six months. Many people do not experience symptoms during this phase, or if they do, they show non-specific symptoms such as fatigue, loss of appetite, and depression.
After six months, approximately 70%-85% of those infected with HCV will fail to clear the virus on their own, or spontaneously, and this is when hepatitis C becomes a chronic or long-term infection. This high rate showcases the importance of regular testing so that treatment, which is highly effective, can start right away.
What are the Symptoms?
Hepatitis C is a tricky virus and often presents in people with no symptoms, giving it its nickname, the silent killer. When a person does show symptoms (symptomatic), they often have:
Fever
Fatigue
Decreased appetite
Nausea, vomiting, and abdominal pain
Dark urine and pale feces
Joint pain
Jaundice
Those who develop symptoms generally have an onset of two to twelve weeks (up to 26 weeks.)
Most people with chronic HCV infections are asymptomatic or have non-specific symptoms such as fatigue and depression. Many of those with chronic HCV infections develop liver diseases that can be severe, such as cirrhosis or liver cancer.
How Soon Can You Test and What Kinds of Tests Are Available?
Anti-HCV seroconversion occurs an average of 8-11 weeks after exposure, although there have been cases of delayed seroconversion in immunosuppressed people, such as those with HIV.
People with a recently acquired acute infection typically have detectable HCV RNA levels as early as 1-2 weeks after exposure to the virus.
https://www.insti.com/hepatitis-c-testing-ultimate-guide/
Blood tests are used to detect HCV infection. Different types are used and include:
Screening tests for antibody to HCV (anti-HCV)
enzyme immunoassay (EIA)
enhanced chemiluminescence immunoassay (CLIA)
Chemiluminescence microparticle immunoassay (CMIA)
Microparticle immunoassay (MEIA)
Electrochemiluminescence immunoassay (ECLIA)
Immunochromatographic assay (rapid test)
Qualitative nucleic acid tests to detect the presence of HCV RNA
Quantitative nucleic acid tests to detect levels of HCV RNA
How Long Do Results Take for HCV Tests?
Test results can take a few days to a few weeks. However, rapid HCV antibody tests offer results much quicker. From one minute (such as with the INSTI® HCV Antibody Test, the world’s fastest and first one-minute HCV antibody test) to 30 minutes for other tests.
How Do Screening Tests Work?
Screening tests, often called HCV antibody tests (or anti-HCV tests), are used to determine if someone has ever been infected with the hepatitis C virus and look for antibodies to the hepatitis C virus in the blood. Antibodies are chemicals released into the bloodstream when someone gets infected.
Screening tests are important as they can help test more people quickly to help people know their status. With their ease of use and quick results, they offer health care providers a way to test more patients and help connect them to care and treatment quickly, including confirmatory HCV RNA tests.
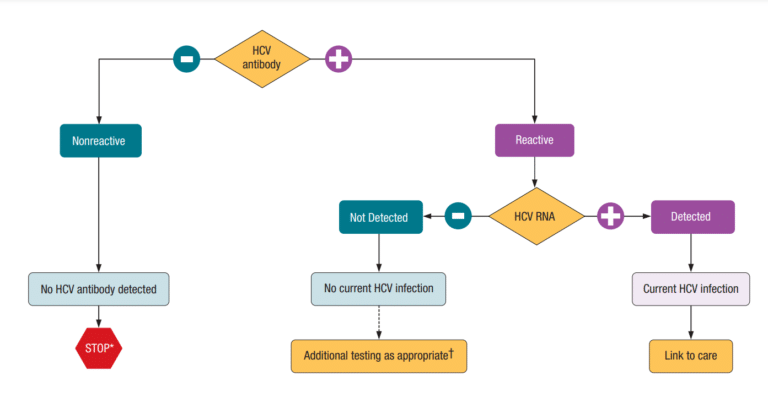
It is important to note that HCV antibody tests are screening tests only and that all positive (or reactive) results will require a follow-up confirmatory HCV RNA test (called a nucleic acid test, or NAT).
What Do HCV Results Mean?
If an HCV antibody test is non-reactive (negative), no antibodies to HCV were found in the blood, which means that the person is not currently infected with the hepatitis C virus. However, if someone thinks they might have been exposed to hepatitis C in the last 6 months, they will need to be tested again.
If an HCV antibody test is reactive (positive), this means the person has been infected with hepatitis C at some point. Once people have been infected, they will always have antibodies in their blood, even if the virus has been cleared or cured. If the result is reactive (positive), an additional confirmatory test is required. This test is called a nucleic acid test (NAT), also called a PCR test, for HCV RNA.
If the NAT for HCV RNA is negative, but the antibody test was positive, this means that the person was infected with HCV, but the virus is no longer in their body because they were either cured with treatment or cleared the virus naturally.
If the NAT for HCV RNA is positive, this means that the virus is in the blood, and the person will need to talk to their doctor to discuss treatment options.
Someone with a positive HCV antibody test, even if they have tested negative for an HCV RNA test, is still at risk for future hepatitis infections, even if they have cleared their current infection. So, it is important to remember to stay safe and take care to reduce risks.
Testing Recommendations
The CDC universal screening guidelines recommend that all adults are tested at least once in a lifetime and for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection is less than 0.1%.
The guidelines vary by province in Canada, but broadscale screening is not recommended for adults who are not at elevated risk. However, those at risk, those from higher prevalence areas, and people with symptoms are advised to test. Additionally, those in the age cohort of 1945-1975 should be tested at least once, as approximately two-thirds to three-quarters of people with hepatitis C in Canada were born in these years.
The WHO recommends a specific approach to testing based on risk and epidemic patterns. They recommend focused or targeted testing for the highest-risk groups, testing among groups with specific past generalized exposure, such as certain birth or age cohorts (such as those in the Baby Boomer generation), and testing in generalized populations with a high HCV prevalence.
WHO Self Testing Guidelines
Recently, the WHO has released recommendations on HCV self-testing as an additional approach to hepatitis C testing services. As part of its goal to eliminate HCV as a public health threat by 2030 in its Global health sector strategy on viral hepatitis, the WHO aims to diagnose 90% of those with HCV and treat 80% of those diagnosed. To help reach this target, they have released guidelines considering HCV self-testing to help increase access to testing and, thus, treatment.
Is There Treatment for Hepatitis C?
Hepatitis C has treatments that can cure most people (over 90%) regardless of their HCV genotype with oral medication (therapy). The medicines recommended are pan-genotypic direct-acting antivirals (or DAAs) for people over 12 years old. DAAs can cure most people with HCV, and treatment is generally short (approximately 8-24 weeks), depending on the absence or presence of cirrhosis.
These medications are often expensive in many high-and-upper-middle-income countries. However, in many lower-middle-income countries, prices have dropped due to increased generic versions of these medications.
And while access is improving, it remains too limited to help end HCV as a public health threat. Of the approximately 58 million people living with HCV, only an estimated 21% or 15.2 million people know their status. And of those, only 62% (or 9.4 million) have been treated with DAAs as of 2019.
Are There Side Effects for the Treatment Using DAAs?
As with all medications, DAAs can cause side effects, though most are mild and become easier to tolerate as the treatment progresses. However, there are occasions when the treatment side effects are severe enough to stop treatment, though this is rarer.
Some of the side effects include but are not limited to:
Fatigue and weakness
Flu-like symptoms
Headaches
Nausea and loss of appetite
Sleep problems or insomnia
Joint pain and muscle aches
Where Can I Get Treatment?
Treatment access will vary depending on the country or region you live in. The best thing to do is speak to your doctor or healthcare provider to understand what treatment is right and how to access it.
There are great resources available in different countries to learn more. In the US, check out the CDC. In Canada, Health Canada or Catie.ca provide great resources. You can check out the NHS for more information in the UK, and the WHO provides information with a global outlook.
Summary
While hepatitis C can lead to serious liver illnesses, including cancer and cirrhosis, it has incredibly effective treatment options that cure over 90% of people living with HCV. This is why testing is so critical for HCV; as there are effective treatment options, the only way to know and get treated is to get tested.
References
Canadian Task Force for Preventative Health Care: https://canadiantaskforce.ca/guidelines/published-guidelines/hepatitis-c/
Catie: https://www.catie.ca/prevention-in-focus/canadian-hepatitis-c-guidelines
Catie: https://www.catie.ca/sites/default/files/hep%20c%20symptoms%20EN%202017%2009%2018_0.pdf
CDC: https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm
EMCDDA: https://www.emcdda.europa.eu/system/files/attachments/11476/9789241549981-eng%281%29.pdf
Global News: https://globalnews.ca/news/4250491/hepatitis-c-screening-canadian-baby-boomers-tested/
Hep C Trust: http://www.hepctrust.org.uk/information/impact-hepatitis-c-liver/progression-hepatitis-c/chronic-phase-hepatitis-c
INSTI Times: https://www.insti.com/hiv-prevention-with-pep-and-prep/
INSTI Times: https://www.insti.com/hcv-a-silent-killer-lurks-among-us/
WHO: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c